|
|
|
Structures and Infrared Spectroscopy of Metal Cation-Borazine Complexes
|
|
|
|
|
|
|
|
|
|
|
|
|
Biswajit Bandyopadhyay
|
|
|
|
Lawrence Berkeley National Laboratory
|
Lawrence Berkeley National Laboratory, 1 Cyclotron Road, Berkeley, California-94720, USA.
|
|
biswajitb@lbl.gov
|
|
|
|
|
|
|
|
|
|

|
|
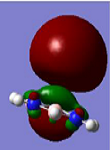
Borazine (B3N3H6) is known as ‘inorganic benzene’ because of a planar B3N3 ring with equivalent B-N distance. The lone pair from N in the ring delocalize to the adjacent p-orbital of B which leads to a conjugated system. Even though metal-benzene complexes have been studied extensively as models for cation- π interactions and organometallic bonding, similar systems with borazine is relatively scarce. Here, we present a density functional study on metal cation-borazine complexes focusing on geometric and electronic structures and their effects on infrared spectra. We have chosen Al, V, Mn, and Zn cations with various d-configurations which provide models for study donor-acceptor complexes. Among these four metal complexes, Al+ and Mn+ prefer to bind to π-cloud on top of the borazine ring. On the other hand, V+ and Zn+ bind to B and N, respectively.
|
|
|
|
|
|
|
|

|
|