|
|
|
Boc-Pyranones as building block towards biologically important Oligosaccharide Natural Pr
|
|
|
|
|
|
|
|
|
|
|
|
|
Sumit O. Bajaj, Ph.D.
|
|
|
|
The Scripps Research Institute
|
Department of Chemistry and Chemical Biology, The Scripps Research Institute, La Jolla, CA 92037, USA
|
|
sobajaj@scripps.edu
|
|
|
|
|
|
|
|
|
|

|
|
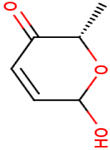
Pyranones play an important role towards the synthesis of carbohydrate containing natural products and are the key building blocks for most of the natural/unnatural oligosaccharides. Boc-Pyranones synthesis is a De Novo approach (i.e, achiral starting material, 2-acetyl furan (1) converted to chiral products) via Noyori asymmetric hydrogenation, Achmatowicz oxidative rearrangement, Upjohn dihydroxylation. Particularly, Boc-protected pyranones have established broad applications towards the synthesis of natural/unnatural products containing carbohydrate motifs (e.g., total synthesis of mezzettiasides, a class of partially acetylated anti-cancer natural products via Pd-/B-dual catalysis and synthesis of cleistriosides/cleistetrosides, a series of rhamno-oligosaccharides).
|
|
|
|
|
|
|
|

|
|